An Intermolecular interaction in binary liquid mixture of Ethyl Aceto Acetate with o, m, p - Xylene: An Volumetric and Viscometric study
Authors
Abstract
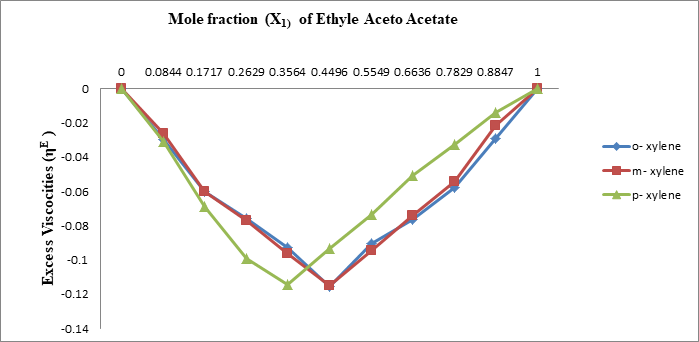
The density and viscosity in binary mixture of Ethyl Aceto Acetate with o, m, p - xylene have been measured over the whole composition range at 303.15K. From these data some of excess viscosity and excess molar volume of o, m, p - xylene in Ethyl Aceto Acetate were calculated using the value of density and viscosity. The results are interpreted in term of molecular interaction between the components of the mixture. It has been observed that molecular interaction existing in the system is highly disturbed by the polar Ethyl Aceto Acetate molecule and depressive type interaction are existing in the system
Article Details
Published
2022-12-05
Issue
Section
Articles
How to Cite
An Intermolecular interaction in binary liquid mixture of Ethyl Aceto Acetate with o, m, p - Xylene: An Volumetric and Viscometric study. (2022). Research and Analysis Journal, 5(12), 08-14. https://doi.org/10.18535/raj.v5i12.372